What is the 505(b)(2) Regulatory Pathway?
What Industry and Bench Scientists Need to Know: Part 3 of 3
How does one begin the process of opening an application for an Investigational New Drug (IND) under the 505(b)(2) New Drug Application (NDA) regulatory pathway? How can you be certain that your proposed product qualifies for the 505(b)(2) classification? How is your product protected under this classification?
With the growing interest in new uses for existing drugs to treat disease, it is important to understand ways to expedite your path to FDA approval. In this series we will discuss how the FDA protects these new drugs with regulatory exclusivities.
If you want to seek FDA review and approval for a new use of an approved drug or have created a new dosage form that has altered kinetics or provides a route of administration or mechanism of drug delivery that advances medical care—then you may wish to understand the 505(b)(2) regulatory pathway. Such new products often contain well-understood active ingredients that are present in existing, approved drug products (reference drugs); so, companies must do 2 things: (a) create a bridge between what is already known about the previously approved reference drug and the novel drug product or indication and (b) position the drug against market competition. The regulatory exclusivities of the 505(b)(2) NDA pathway makes this possible.

Examples of 505(b)(2) candidates include:
- Drugs with new indications
- Drugs with changes in dosage form, strength, formulation, dosing regimen or route of administration
- New combination products
- Prodrugs of an existing drug
- Drug-device combinations
Regulatory Exclusivity
New drugs that are approved under the 505(b)(2) regulatory pathway can be protected from competition in several ways. In addition to patents which typically protect inventions for 20 years after discovery; developers can protect drug products from market competition for up to 7 years after approval. The length of protection is different for each chemical entity. Overall, the protections typically run concurrently, but some may be staggered or stacked end to end. The regulations for new product exclusivity are listed in the United States Code of Federal Regulations at 21CFR 312.108
Regulatory Exclusivity Features for Small Molecules
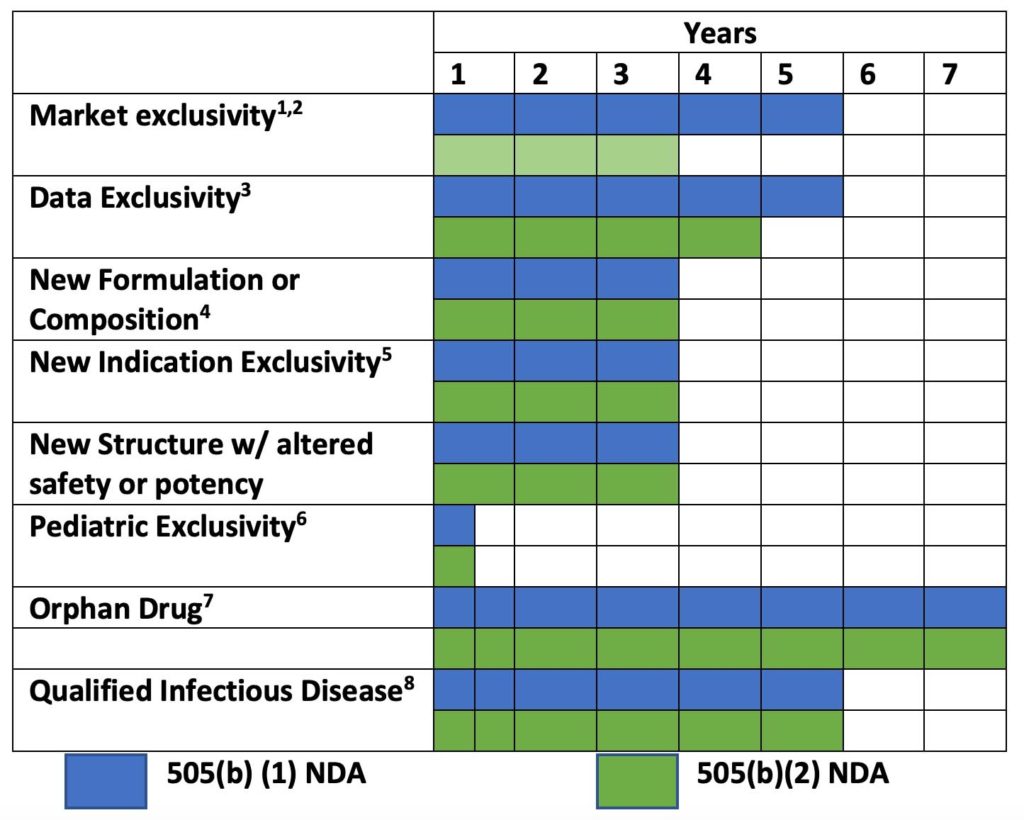
- A 5-year period of market exclusivity is granted to new drug applications for products containing new chemical entities under 505(b)(1) and 505 (b)(2) for the first indication, if patents have not been filed and issued. During this period, no 505(b)(2) application or Abbreviated New Drug application for a generic may be submitted –except if they contain a certification of patent infringement
- A 3-year period of exclusivity is granted for a drug product that contains an active moiety that has been previously approved, when the application contains reports of new clinical investigations (other than bioavailability studies) conducted or sponsored by the sponsor that were essential to approval of the application
- A drug that contains a new chemical entity (i.e., a new active ingredient) that has not been approved for use in another drug may receive 5 years of data exclusivity running from the time of NDA approval. After 4 years the FDA may review a 505(b)(2) application for the same ingredient
- New formulation or composition exclusivity of 3 years is granted to new drug applications for products containing new chemical entities under 505(b)(1) and 505 (b)(2). The FDA may accept a 505(b)(2) submission that references the new composition during the 3-year period.
- Some experts believe that products that are characterized only by a new indication without change in structure, formulation or delivery are not likely to receive 3 years exclusivity because of off-label use from readily available generics
- Pediatric exclusivity (an additional 6 months) is allowed when the FDA requests that the applicant conduct pediatric studies and such studies are completed.
- FDA designates an Orphan Drug as one that treats a disease or condition that affects fewer than 200,000 people in the US. Under this designation the FDA cannot approve another application for the same drug or disease for 7 years (some exceptions apply).
- Exclusivity for an antibacterial or antifungal drugs that are intended to treat serious, life threatening, or resistant pathogens is extended an additional 5 years over other exclusivities under 505(b)(1) and 505 (b)(2).
References:
Congressional Research Service Drug Prices_ The Role of Patents and Regulatory Exclusivities_2021. Accessed at https://crsreports.congress.gov/product/pdf/R/R46679
Authors:
Kenneth Duchin, PhD is an experienced clinical pharmacologist with extensive drug development experience in a broad range of therapeutic areas (e.g., oncology, urology, diabetes, CNS, CV, GI). He has led several registration programs and authored regulatory documents to support regulatory filings.
Dr. Cheryl Rowe-Rendleman is a regulatory and clinical development expert with over 15 years’ experience in pharma. She founded Omar Consultants and has served as CEO and Managing Consultant of the company since 2005. Omar Consulting Group, LLC helps clients reach the goal of executing a successful clinical /regulatory strategy that will be adequate for regulatory authorities.