Strategic Clinical Design and Regulatory Meetings
Target Product Profiles
The Target Product Profile (TPP) is the living document that is at the core of strategic clinical design:
Provides the scope of development for a therapeutic
Lists the known attributes of a clinical development plan and describes the gaps that must be filled before successful funding and/or launch of a product
Provides a format for discussions between a sponsor and the FDA that can be used throughout the drug development process
Describes the value and net worth of your development program to potential investors and stakeholders
The TPP embodies the notion of beginning with the goal in mind. Omar Consultants are experienced in development of TPPs
Source: https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm080593.pdf
Protocol Validation
Omar Consulting and its CRO partners will help you validate your protocol. These services include:






Sometimes even a well-validated protocol must be amended. When this happens, Omar Consulting will help you amend your protocol and provide you with submission ready copies for your investigational review board.

Subpopulation Optimization
Omar Consulting partners specialize in bridging studies and extrapolation of data to special subpopulations.
Omar Consulting helps clients with global studies to determine whether ethnic bridging studies may be necessary through adequate characterization of:
- PK/PD
- Efficacy and Safety
- Dose-Response
Strategic Design Elements and Parallel Activities for Drugs and Biologics
Omar Consulting lends expertise to help develop strategic approaches to design of clinical programs
for drugs and biologics from preclinical research thru market launch.
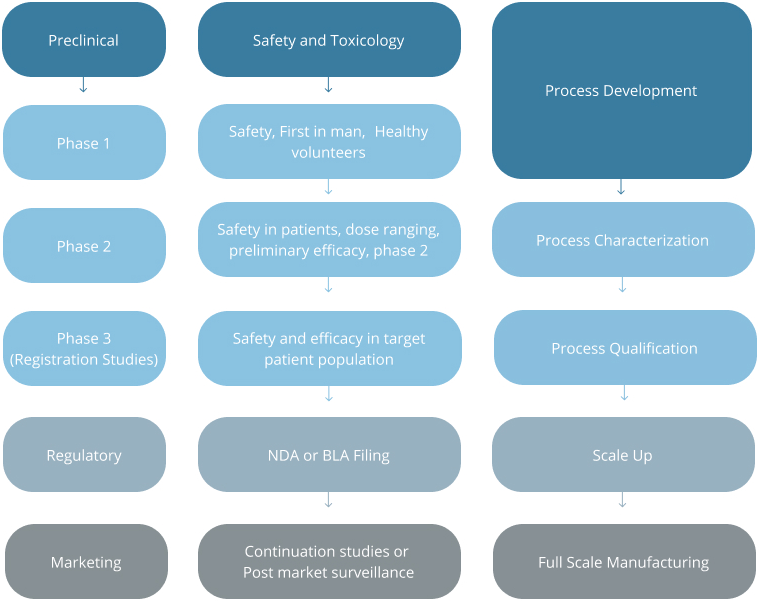
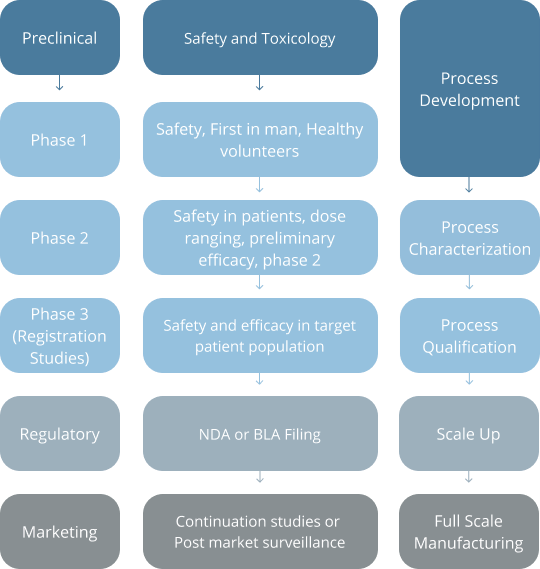
At the end of Phase 3 trials a New Drug Application (NDA) or, in the case of a biologic, a Biologics License Application is usually submitted to the regulatory agency, with a request for approval to market a specific compound for the indications specified in the application.
Source: Modern Methods of Clinical Investigation, Institute of Medicine (US) Committee on Technological Innovation in Medicine; Gelijns AC, editor
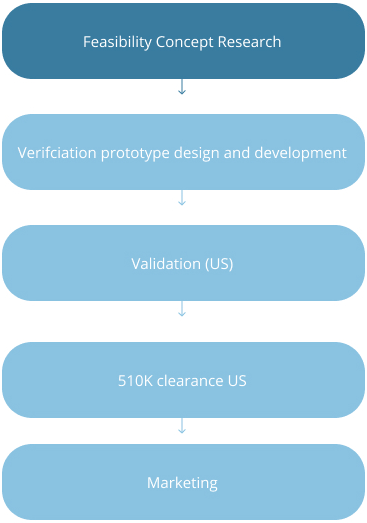
Strategic Stages of Device Development in US and EU
US: Class I and II Devices
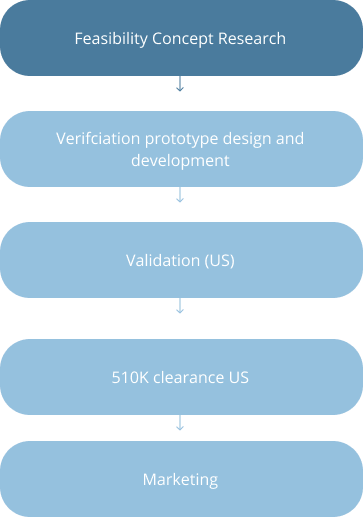
EU: Class I and II Devices
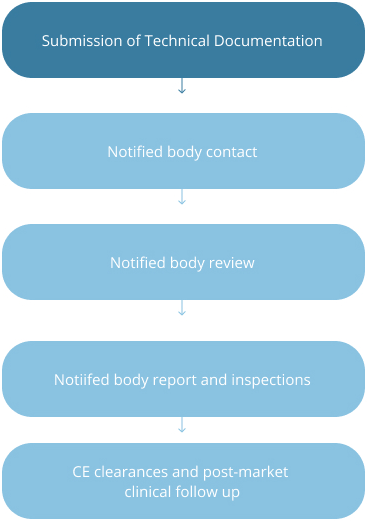
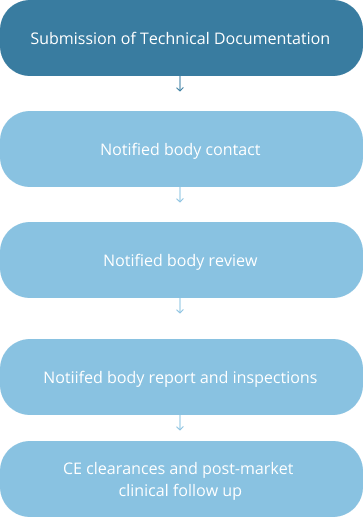
FDA Meetings
There are four types of formal meetings that can occur between requesters and FDA staff:
Type A, Type B, Type B (end of phase (EOP), and Type C
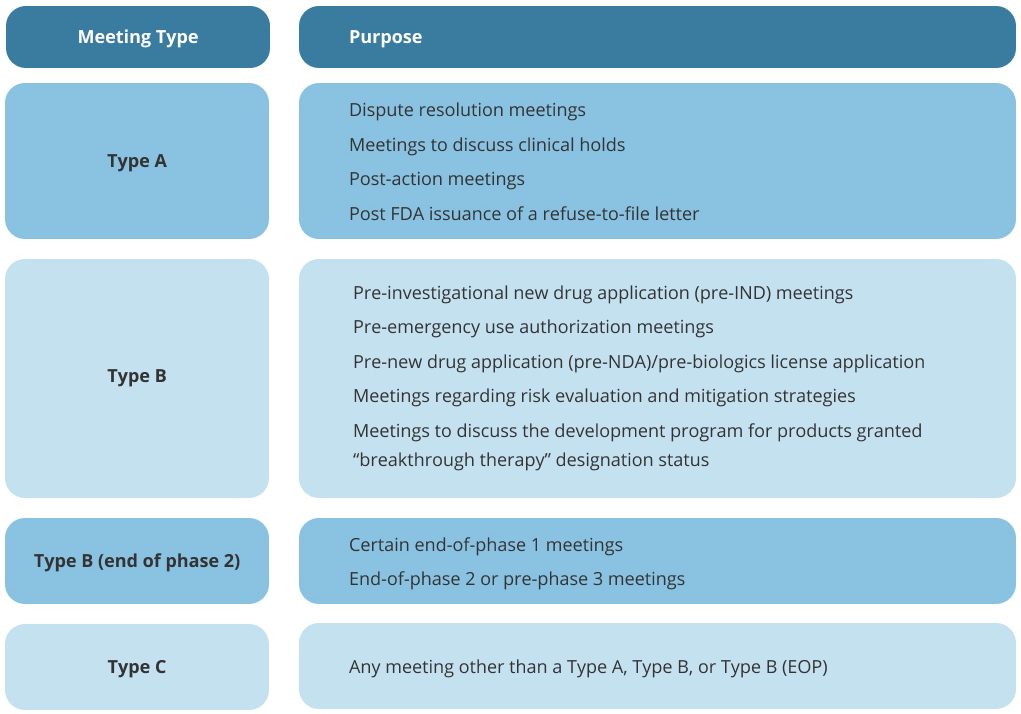
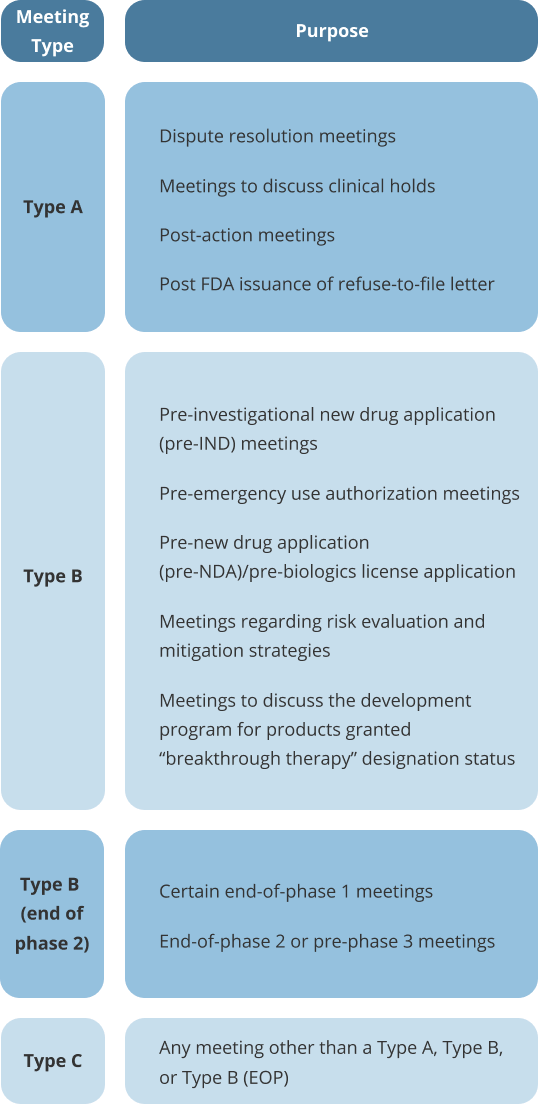
Omar Consultants will help you prepare for your next FDA meeting
Source: https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM590547.pdf